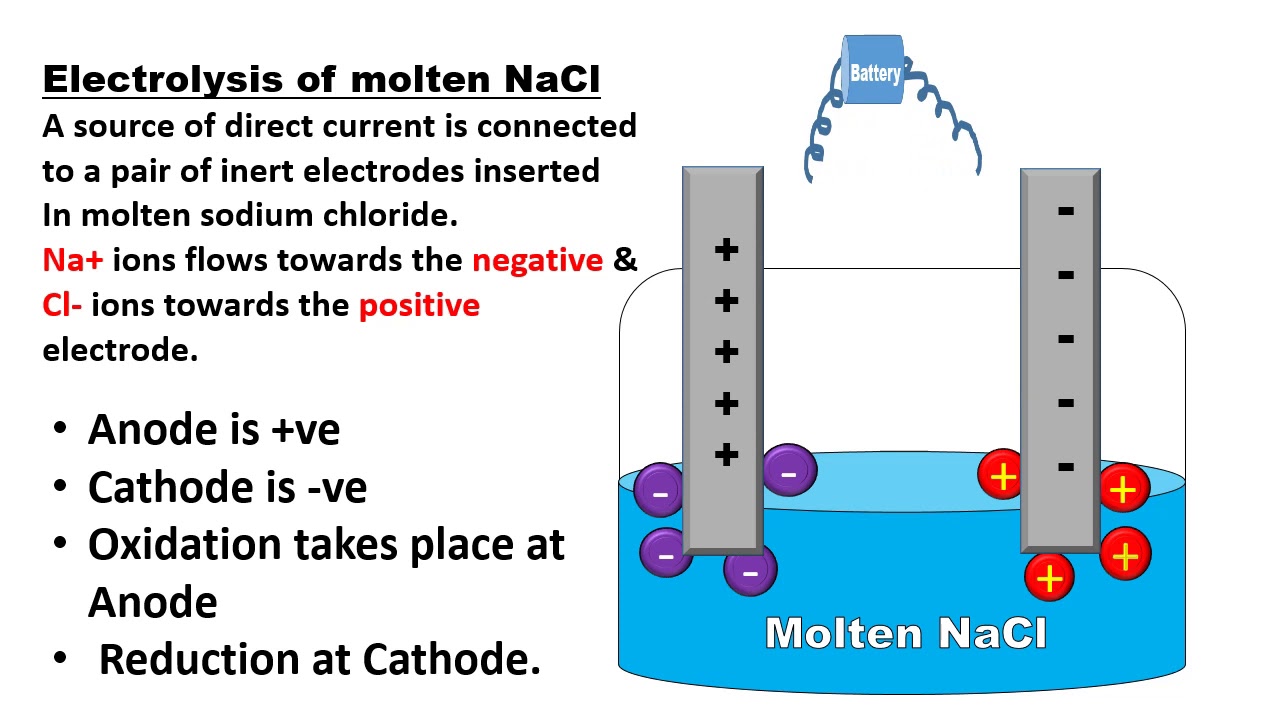
Anode and Cathode in Electrolysis
Ags NO3 AgNO3aq e Therefore Ag will deposit at the cathode and dissolve at anode. It depends on its oxidationreduction potentials.

Look4chemistry Electrolytic Cell Electrochemistry Cell Positive And Negative
Ag e Ag.
. It is the electrodemetal plate that is connected to the positive terminal of the cell. The so-called anode effects and polarization and to avoid the difficulties related to the carbon anode 3-5. The reaction takes place in a unit called an electrolyzer.
Electrolysis Of Copper Sulfate Solution With Impure Copper Anode And Pure Copper Cathode Copper Purification Technology Educat Pure Products Copper Solutions. Electrolysis is the process of using electricity to split water into hydrogen and oxygen. An anode is an electrode by which the conventional current enters into a polarized electrical device.
Anode and cathode are defined by the flow of current. Par Août 30 2022 polywood braxton sofa 25 gallon steel drums for sale Août 30 2022 polywood braxton sofa 25 gallon steel drums for sale. What will happen during the.
Best anode and cathode for electrolysis. OH- through the electrolyte from the. The electrolysis of acidulated water is considered to be an example of catalysis.
Answer 1 of 3. The cathode is negatively charged to remember how the cathode is negatively charged consider caThode T as a negative so it is easy to remember carhode as a negatively. Fitbit luxe charging cable.
The terms were coined in 1834 by William Whewell who derived the words from the Greek word Kathodes which means. Oil and gas news from 19 to 25 June 2017 June 27. The anode positive electrode is made from impure copper and the cathode negative electrode is made from pure copper.
Galvanic or electrolysis is a chemical process. Black rattan tray round. However limited attention has been paid to the study of cathode.
There are three modalities used today that fall under the heading of electrolysis. The current produces a chemical reaction in the hair follicle. Posted on August 30 2022 by.
Anode and Cathode. Answer 1 of 3. Facebook page opens in new window YouTube page opens in new window Twitter page opens in new window Linkedin page opens in new window.
Anode is electron deficient and hence the negative ions are. You can use pretty much any metal for the cathode the electrode that evolves hydrogen but the anode is more difficult. Best anode and cathode for electrolysis.
B During the electrolysis of copper II sulphate solution using platinum as cathode and. The product of electrolysis of concentrated aqueous sodium chloride are sodium hydroxide hydrogen gas and chlorine gas. In contrasts with a cathode an electrode by which conventional current.
During electrolysis the anode loses mass as. An aqueous solution of Na_2SO_4 is electrolysed using Pt. Trick to find products of electrolysis at cathode and anode Electrochemistry Class 12Test your self solution linkhttpsyoutuben8DktAvRGo8.
You need something that will resist corrosion while. Here the anode is positive and cathode is the negative electrode. The reaction at the anode is.
For example in the electrolysis of a NaCl solution in water the reduction potential of water to H2 is -083 V while. The reaction at the anode is oxidation and that at the cathode is reduction. Best anode and cathode for electrolysis.

Electrolysis Process On Passing Electric Current The Cations Move Towards The Cathode And Get Deposited Piscinas De Agua Salada Escuela De Natacion Piscinas

Electrolysis Chemical Changes Electrochemistry Energy

Electrolysis Of Copper Sulfate Solution With Impure Copper Anode And Pure Copper Cathode Copper Purification Technology Educat Pure Products Copper Solutions

What Is Electrolytic Cell Electrochemistry Chemistry Basics Chemistry
0 Response to "Anode and Cathode in Electrolysis"
Post a Comment